A team led by Prof. SONG Li from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) proposed the concept of intercalant-induced V t2g orbital occupation and developed an NH4+-intercalated vanadium oxide (NH4+-V2O5) for high-performance aqueous zinc-ion batteries (ZIBs). This work was published in PNAS, entitled “Intercalant-induced V t2g orbital occupation in vanadium oxide cathode toward fast-charging aqueous zinc-ion batteries”.
ZIBs, due to their safety, non-toxicity, and high theoretical capacity, have become one of the most promising sustainable energy storage technologies. Among various electrode materials for ZIBs, vanadium oxides have been widely studied as cathode materials for ZIBs due to their flexible crystal structure and multivalence of vanadium. Based on the ion or molecular pre-intercalation strategy, the problems of insufficient lattice space and low electronic conductivity of cathode materials can be effectively solved, thereby further improving battery performance. However, current research on intercalation cathode materials mostly focuses on the enhancement of capacity by interlayer space expansion. Therefore, it is important to develop advanced in-situ characterization techniques, which assist in studying the intrinsic structural variation of electrode materials induced by intercalants from the perspective of atomic orbitals This will become the key to the design of high-performance cathode materials in the future.
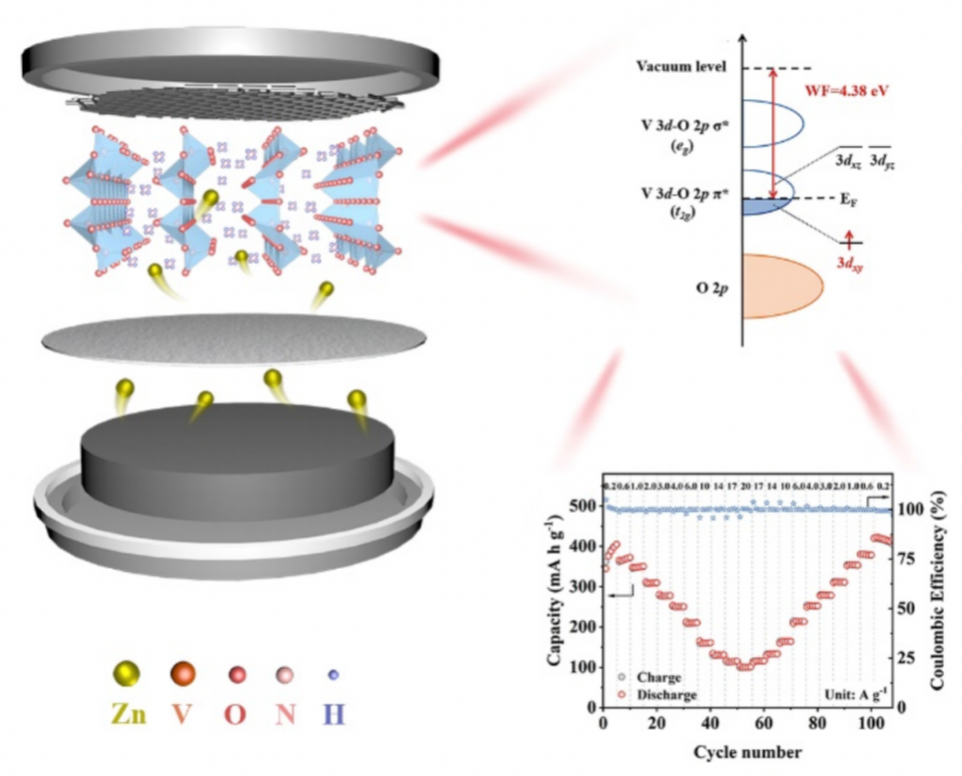
V t2g orbital occupation mechanism applied to NH4+-V2O5. (Image by SONG Li et al.)
In this work, researchers introduced a variety of in-situ and ex-situ synchrotron radiation spectroscopy techniques, deeply revealing the change of V 3dt2g orbital occupation in V2O5 after the intercalation of NH4+, as well as the reversible evolution law during the charge and discharge process. They found that NH4+ intercalation largely induced the structural distortion of the V-O bond, which further led to a rearrangement of the electronic structure and facilitated the occupation of the 3dxy vacancy state in the V t2g orbital. The V t2g orbital occupation greatly improved the electrical conductivity of the material. Besides, NH4+ intercalation resulted in the broadened layer spacing, which significantly accelerated the electron transfer and Zn-ion migration, realizing the ultra-high multiplicity performance of the zinc ion battery.
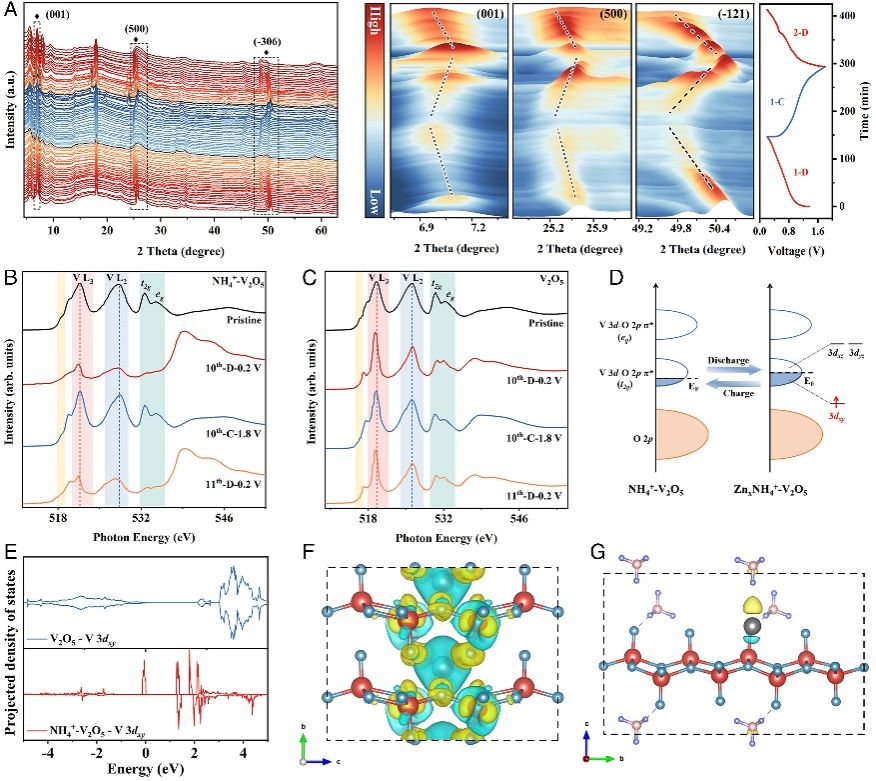
Analysis of the energy storage mechanism of NH4+-V2O5 applied to high-performance ZIBs cathode materials. (Image by SONG Li et al.)
The experimental results showed that the specific capacity of the ammonium intercalated vanadium pentoxide (NH4+-V2O5) cathode material remains the rate capability at 101.0 mA h g-1 at a current density of 200 C, with a charging time of 18 s.
This study provides a better understanding of the mechanism of storing Zn2+ in intercalated V2O5 materials and lays a foundation for the design of high-performance intercalated cathode materials of ZIBs.
Paper link: https://www.pnas.org/doi/10.1073/pnas.2217208120
(Written by HUANG Rui, edited by WANG Zhaokun, USTC News Center)
